🇺🇲 This page is intended for US healthcare professionals only


womed leaf®
Resorbable Adhesion Barrier
womed leaf® is the
FIRST FDA-APPROVED
medical device for women with Asherman syndrome
The Womed Leaf® Resorbable Adhesion Barrier is intended to reduce the reoccurrence and severity of post-surgical adhesion formation inside the uterus. It is indicated for adult women undergoing hysteroscopic surgery for symptomatic moderate to severe intrauterine adhesions.


Principle

About intrauterine adhesions or Asherman syndrome

Intrauterine adhesions (IUA) or synechiae are fibrous strings at opposing walls of the uterus leading to partial or complete obliteration of the cavity. They are also be referred to as Asherman syndrome. They occur as the consequence of surgery in the uterine cavity and are the major long term complications of operative hysteroscopy.
They are responsible for menstrual disorders, infertility and obstetrical complications such as abnormal placentation. They are associated with miscarriages and retained product of conception with a reported incidence as high as 20% following dilation and curettage for those conditions (1, 2) and up to 45% after myomectomy (3,4).
Removing adhesions, or adhesiolysis, is performed by hysteroscopy. The recurrence rate after intrauterine adhesiolysis is between 42% and 75% in case of severe adhesions or Asherman syndrome (5). Women with Asherman syndrome who try to conceive often need to go through multiple interventions, without any assurance of achieving pregnancy.
Surgical adhesions can be prevented by separating the adjacent injured tissues at the beginning of the healing phase. While adhesion barrier products exist for abdominal or pelvic surgery like absorbable films or gels, none of them is adequate for intrauterine use, leaving women's uterus unprotected after an intervention.
Insertion
At the end of the adhesiolysis surgical procedure, Womed Leaf is inserted through the cervix like an IUD and the uterine film is released.


Self deployment
The film swells and expands in contact with uterine fluids
Physical barrier
By keeping the walls apart, Womed Leaf acts as a physical barrier.


Natural discharge
The uterine film breaks-up, dissolves and is naturally discharged without any intervention of a healthcare professional.
Why Womed Leaf®?

Quick insertion
Womed Leaf® is inserted through the cervix with a flexible inserter, similar to an IUD in less than 2 minutes.

No removal required
The uterine film fragments, dissolves and is naturally discharged through the cervix without any intervention of a healthcare professional.

Painless protection
Womed Leaf® film does not cause pain: it is soft 0.02" thick and is available in 3 sizes for uterine cavity length up to 10 cm.
Clinical evidence
Womed® is committed to evidence-based medicine and is investing in clinical research to demonstrate the medical value of its products and establish them as new standards of care.
Womed Leaf® clinical program
Womed Leaf®'s clinical evaluation is part of a large study program. The program aims to bring solid scientific evidence to the ObGyn community and benefit women in their fertility projects. It is designed in phases. :
-
PREG1: European study to validate safety after hysteroscopic myomectomy (completed)
-
PREG2: International study to evaluate effectiveness after adhesiolysis (completed)
PREG1 pilot clinical study
PREG1 (NCT04381728) is a prospective, multi-center, single arm clinical study that aimed to evaluate the safety of Womed Leaf® after hysteroscopic myomectomy and its efficacy in preventing intrauterine adhesions at second look hysteroscopy. This first-in-human study demonstrated that Womed Leaf® is well tolerated by the patients. There were three adverse events but none were designated to be device related. Out of the 23 patients, 20 were free of adhesion at second look hysteroscopy (87%); out of the three reported adhesions, none were classified as severe. The performance of the Womed Leaf® inserter was 100% successful from the first attempt with a device manipulation duration from insertion to withdrawal estimated at less than 2 minutes.
The study manuscript is published in JMIG (Journal of Minimally Invasive Gynecology).
PREG2 pivotal clinical study
PREG2 (NCT04963179) is a multicenter, international (outside the United States), double-blind, randomized, controlled superiority pivotal trial stratified by IUA severity and site, involving 163 women with moderate or severe IUAs, where hysteroscopic adhesiolysis followed by insertion of Womed Leaf® was compared to adhesiolysis alone..

Safety results
The analysis of safety was based on the safety cohort of 163 patients. No serious adverse event was reported during the 6 weeks follow-up period of the trial. Additionally, no polymer remnant or endometrial abnormalities were reported during the trial. The patient-reported outcomes, collected at the 6-week follow-up visit showed Womed Leaf® does not generate additional pain or discomfort (including during its discharge) compared to adhesiolysis without barrier.
Effectiveness results
The analysis of effectiveness was based on the 153 evaluable patients at the 6-week time point. The trial met its primary endpoint: the reduction of AFS score between baseline and second-look hysteroscopy was statistically significantly higher in the Womed Leaf® group than in the control group.
Conclusion
The PREG2 randomized controlled trial demonstrated that Womed Leaf® significantly reduced the severity of intrauterine adhesion after hysteroscopic adhesiolysis compared with no prevention method and that Womed Leaf® has an acceptable safety profile.
The study results were published in Fertility and Sterility.
We could help you and your patients
Womed Leaf® is approved for sale by the United States Food and Drug Administration
The Womed Leaf® Resorbable Adhesion Barrier is intended to reduce the reoccurrence and severity of post-surgical adhesion formation inside the uterus. It is indicated for adult women undergoing hysteroscopic surgery for symptomatic moderate to severe intrauterine adhesions.
Womed Leaf® will be available in the United States in early 2026.
Federal law restricts this device to sale by or on the order of a licensed healthcare practitioner.
Important risk information
Womed Leaf® is contraindicated for patients with known hypersensitivity to Womed Leaf® or any of its components.
The device must not be used in patients with infection or contamination of the procedure site.
Womed Leaf® must be used according to the instructions for use. Failure to follow the insertion instructions may result in an incorrect positioning of the Womed Leaf® in the endometrial cavity and premature discharge.
Womed Leaf® is designed and intended for single use only and must not be re-used or re-sterilized. Reuse of the device may lead to infection leading to endometritis, salpingitis, or pelvic peritonitis.
In case of suspicion of thin uterine wall, extra care should be taken due to the higher perforation risks.
Adverse effects may include allergic reaction, including pruritus and vulvovaginal pruritus, infection (rash, fever), persistent pelvic pain, urethral pain or bleeding.
Womed Leaf® should not be used in pregnancy.
Womed Leaf® should not be used if the temperature indicator on the box is black or if the protective pouch is damaged or unintentionally opened before use as sterility may be compromised.
The safety and effectiveness of Womed Leaf® has not been established in patients with an abnormal uterine cavity (i.e., unicornis, bicornis, septate, duplex).
The safety and effectiveness of Womed Leaf® has not been established in patients with a history of endometrial ablation.
The safety and effectiveness of Womed Leaf® has not been established in patients with a uterine length >10 cm.
The safety and effectiveness of Womed Leaf® has not been established following hysteroscopic procedures other than adhesiolysis of moderate to severe adhesions.
Uterine film placement can be verified post-procedure by ultrasound imaging.
During inserter preparation and insertion, keep plunger mark visible to avoid premature film release.
Patients should be informed to avoid pregnancy during the first complete menstrual cycle subsequent to the treatment with Womed Leaf®
Do you need help?
Instructions for use
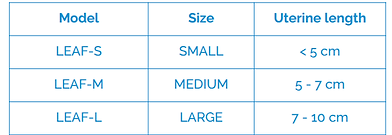
Select the appropriate device's size based on the depth of the uterine cavity.
Learn the step by step insertion procedure with the IFU video.
References
Womed Leaf publications
-
Effectiveness of degradable polymer film in the management of severe or moderate intrauterine adhesions (PREG-2): a randomized, double-blind, multicenter, stratified, superiority trial - Fernandez et al (2024) Fert and Ster
-
First Use Of A Novel Barrier Film In An Infertile Woman With Severe Asherman Syndrome - Sroussi et al (2022) JMIG
-
Hysteroscopic and ultrasound evaluation of a novel degradable polymer film for the prevention of intrauterine adhesion formation after hysteroscopic surgery - Zizolfi et al (2022), European Journal of Obstetrics and Gynecology
-
Safety and efficacy of a novel barrier film to prevent intrauterine adhesion formation after hysteroscopic myomectomy: The PREG1 clinical trial - Weyers et al (2021). Journal of Minimally Invasive Gynecology.
-
In Vivo Evaluation of the Efficacy and Safety of a Novel Degradable Polymeric Film for the Prevention of Intrauterine Adhesions. Huberlant et al., Journal of Minimally Invasive Gynecology. 2021, vol. 28, no 7, p. 1384-1390
-
Preliminary design of a new degradable medical device to prevent the formation and recurrence of intrauterine adhesions - Leprince et al., Communications Biology (2019) 2:196
-
A new bioabsorbable polymer film to prevent peritoneal adhesions validated in a post-surgical animal model - Allègre et al., (2018) PLoS ONE 13(11): e0202285.
Other publications
-
Salazar et al. A comprehensive review of Asherman’s syndrome: causes, symptoms and treatment options Curr Opin Obstet Gynecol 2017, 29:249–256
-
Hooker et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome Human Reproduction Update, 2014;20(2)262–278
-
Taskin et al. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. The Journal of the American Association of Gynecologic Laparoscopists 2000;7(3):351–4
-
Guida et al. Effectiveness of auto-crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: a prospective, randomized, controlled study. Human Reproduction 2004;19(6):1461-64
-
Warembourg S et al. Prevention and treatment of intrauterine synechiae : Review of literature. J Gynecol Obstet Biol Reprod. 2015 Apr ; 44 :366-79.
